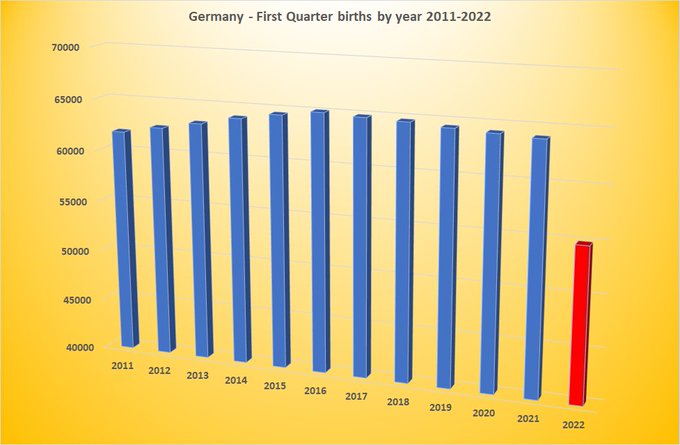
-
25th June 2022, 04:40 PM
#3321
Iridium

-
-
25th June 2022, 05:12 PM
#3322
Iridium

Re: Coronavirus
This is my second posting featuring Dr. John Jacobs. This is bombshell data. YouTube probably will spike this video very soon. This a few minutes more than the 1 minute trailer I posted earlier but full interview is still behind a pay wall on Epoch TV.
This video is premiering live right now but in a few minutes, the replay should be available with the same url. This was a VERY short video like maybe 10 minutes.
https://www.youtube.com/watch?v=DK7IVxa5l8s
87% of Trial Data Hidden from Medical Journals; Fmr FDA Director: Not Our Job to Correct Articles
Facts Matter with Roman Balmakov
782K subscribers
Content:
-
-
26th June 2022, 01:18 AM
#3323
Iridium

Re: Coronavirus
Dachsie comment:
Congressman Scalise held up a sign in congress a few days ago with the number 434 on it, saying Dr. Fauci has not testified on the House floor in over a year, 434 days.
Just pointing out that Fauci is hoping to slither out of his position very soon. He plans to just take the money and run.
______________________
Top House Republican: 'Why Are They Hiding Dr. Fauci?'
https://www.youtube.com/watch?v=7bJeMyssEtQ
6:47 video runtime
Forbes Breaking News
Jun 23, 2022
At today's House Select Committee of the Coronavirus, Rep. Steve Scalise (R-LA) called out the absence of Dr. Anthony Fauci from House hearings.
https://youtu.be/7bJeMyssEtQ?t=140
_____________________
https://www.republicanwhip.gov/news/...-hiding-fauci/

WASHINGTON, D.C. — House Republican Whip and Ranking Member of the Select Subcommittee on the Coronavirus Crisis Steve Scalise’s (R-La.) opening statement at today’s Select Subcommittee’s hearing calls out Democrats for refusing to investigate the Biden Administration’s horrific track record during the COVID-19 pandemic. Whip Scalise criticizes Democrats on the Select Subcommittee on the Coronavirus Crisis for failing to hold a hearing with President Biden’s Chief Medical Advisor, Dr. Anthony Fauci in over a year. Additionally, Whip Scalise urges Select Subcommittee Democrats to hold a hearing about the origins of the coronavirus and investigate claims that the Centers and Disease Control and Prevention (CDC) allowed teacher union bosses to re-write critical portions of the Biden Administration’s school reopening guidance, which effectively kept thousands of schools closed when they could have reopened.
“Thank you, Mr. Chairman.
“I would like to welcome Dr. Birx. Thank you for being here today.
“And thank you for your four decades of service to this country, including 29 years in the U.S. Army.
“Like Groundhog Day, my Democrat colleagues will use today to continue their political vendetta against the former Trump Administration while continuing to cover up the many failures that have occurred with respect to COVID-19 under the Biden Administration.
“It is our job to perform congressional oversight over the administration. That’s why Republicans on this Subcommittee have focused on serious issues like the CDC’s school reopening guidance and the emails that prove the Biden Administration colluded with union bosses to keep millions of American children locked out of their classrooms.
“We now know that the social isolation and closed schools caused by the COVID lockdowns resulted in serious mental health issues and dramatic learning loss for millions of America’s kids. The last time I tried to talk about this incredibly important issue, the Chairman urged us all to not look backward, stating:
“‘I would hope we won’t spend all of our time today talking about yesterday. I’m concerned about tomorrow and the day after.’ (14:53)
“He went on to say, ‘All of us can spend the rest of our lives talking about what happened before COVID-19, or we can spend a little time trying to figure out how best to move forward from whatever mistakes may have been made, whoever may have made them, or we can spend all of our time assigning blame.’ (47:39).
“But here we are today, having yet another hearing with a witness to discuss things that happened more than two years ago while working for the former president.
“No hearing with Biden’s first COVID-19 response coordinator and Dr. Birx’s immediate successor, Jeff Zeints.
“No hearing with Biden’s current coordinator, Dr. Ashish Jha.
“And no hearing in more than a year with Biden’s Chief Medical Advisor, Dr. Fauci.
“Of course, I welcome Dr. Birx’s testimony today, but I would be remiss if I didn’t point out the irony of the Chairman’s past statements against talking about the past. It is noteworthy that this Subcommittee would focus on the events of years ago while denying our ability to address the failures of the Biden Administration that are still occurring to this day.
“More Americans died from COVID during President Biden’s first year than Trump’s, even with multiple vaccines. And we are currently dealing with the serious impact COVID lockdown policies have had on millions of kids. I’m worried the effects will last for years to come, jeopardizing their future ability to achieve their dreams.
“We need a proper investigation into the origins of COVID. I have asked the Chairman for this many times and yet we still have not had a hearing to answer this question that millions of Americans keep asking.
“Again, begs the question of why this Select Subcommittee on Coronavirus refuses to hold a hearing on the origins of COVID-19 when the World Health Organization, and a growing list of leading experts in the scientific community all deem it worthy of investigation.
“Dr. Jeffrey Sachs of Columbia University who himself thinks this came from a lab said COVID-19 is ‘a blunder of biotech not a natural spillover.’
“And now it is being reported that Dr. Tedros, the head of the WHO, is quietly confiding to officials that he believes this pandemic originated in a lab in China.
“I wonder if Dr. Sachs and Dr. Tedros are—to use the Chairman’s own words—‘us[ing] the issue of the origin of the virus to shift accountability from President Trump to Dr. Anthony Fauci,’ as he accused Republicans of doing.
“More than one million Americans have died from COVID. We should understand how this virus started not only because we owe it to those that have died but also to protect against future pandemics. China was doing risky research in a lab that likely caused this pandemic. Did China lie to the world about the virus and how it spread? These are questions we should absolutely know the answers to.
“To that end, I know Dr. Birx has told us before that the origins of the virus are detectable if China would be willing to share the earliest sequences of the virus. And Dr. Birx has previously said that early statements from scientists, scientific publications, and the media downplaying the lab leak were premature and not based on data.
“I’m glad we’ll get the opportunity to hear what she has to say about China’s involvement and how the U.S. and the rest of the world were misled by China and the World Health Organization.
“I also look forward to hearing Dr. Birx’s suggestions for reforming the CDC. Over the course of the pandemic, Americans have lost trust in what was once a premier public health organization. Their failures must be confronted in order for that trust to be restored.
“Thank you, Mr. Chairman. I yield back.”
-
-
26th June 2022, 05:36 AM
#3324
Iridium

Re: Coronavirus
Dachsie comment:
Even though Dr. Debby is no longer in government, she's still able to dissemble with the best of them.
I was able to listen the full 6 minutes though use of the word "utilize" usually makes me quit.
__________________________
https://www.youtube.com/watch?v=-GE62rNmXfA
Should The United States Be In The World Health Organization?': Jim Jordan Grills Dr. Birx
6:34 video runtime
Jun 23, 2022
Forbes Breaking News
At today's House Select Committee on the Coronavirus, Rep. Jim Jordan (R-OH) questioned Dr. Deborah Birx.
-
-
26th June 2022, 07:06 AM
#3325
Iridium

Re: Coronavirus
Dachsie comment:
With the release of more pages of documents at the first of each month, it is made ever more clear to us just how very corrupt Pfizer and the FDA are.
_________________________
https://childrenshealthdefense.org/d...9-9b303d83b1db
06/21/22
•
COVID › News
Pfizer Classified Almost All Severe Adverse Events During COVID Vaccine Trials ‘Not Related to Shots’
The case reports included in Pfizer clinical trial documents, released June 1 by the U.S. Food and Drug Administration, reveal a trend of classifying almost all adverse events — and in particular severe adverse events — as being “not related” to the vaccine.
By
Michael Nevradakis, Ph.D.

[SIZE=3]The latest release by the U.S. Food and Drug Administration (FDA) of Pfizer-BioNTech COVID-19 vaccine documents reveals numerous instances of participants who sustained severe adverse events during Phase 3 trials. Some of these participants withdrew from the trials, some were dropped and some died.
The 80,000-page document cache includes an extensive set of Case Report Forms (CRFs) from Pfizer Phase 3 trials conducted at various locations in the U.S., in addition to other documentation pertaining to participants in Pfizer-BioNTech vaccine trials in the U.S. and worldwide.
The FDA on June 1 released the documents, which pertain to the Emergency Use Authorization (EUA) of the vaccine, as part of a court-ordered disclosure schedule stemming from an expedited Freedom of Information Act (FOIA) request filed in August 2021.
Public Health and Medical Professionals for Transparency (PHMPT), a group of doctors and public health professionals, submitted the FOIA request.
CRFs show deaths, severe reactions to the vaccines during Phase 3 trials
The CRFs included in this month’s documents contain often vague explanations of the specific symptoms experienced by the trial participants.
They also reveal a trend of classifying almost all adverse events — and in particular severe adverse events (SAEs) — as being “not related” to the vaccine.
For example:
A female in her early 50s (randomization number 86545) who participated in the trial at the Sterling Research Group in Cincinnati, Ohio, died of an apparent myocardial infarction on Nov. 4, 2020. She had received two doses of the vaccine, on Sept. 10 and Sept. 29, 2020.
The patient had a medical history of chronic obstructive pulmonary disease, hypertension, hypothyroidism, osteoarthritis of the knees and attention deficit disorder. Her death was listed as “not related” to the vaccine, and was instead attributed to “hypertensive cardiovascular disease.”
A female in her late 50s (randomization number 220496), who participated in the trial at Cincinnati Children’s Hospital Medical Center, died of cardiac arrest on Oct. 21, 2020. Her death, however, was indicated as “not related” to her vaccinations (which occurred on July 30, 2020, and Aug. 20, 2020) as it “occurred 2 months after last receipt of study agent,” according to her CRF.
The participant’s medical history included obesity, placement of a gastric sleeve, gastroesophageal reflux, sleep apnea, supraventricular tachycardia, hypothyroidism, depression and asthma.
A male in his mid-60s (randomization number 221076) who participated in the trial operated by the Texas-based Ventavia Research Group died of an apparent myocardial infarction on Nov. 28, 2020. He had received the two doses of the vaccine on July 31, 2020, and Aug. 19, 2020.
The participant had a medical history that included a previous myocardial infarction, high blood pressure, high cholesterol, anxiety, bilateral hip pain, type 2 diabetes, fluid retention, angina (intermittent), restless leg syndrome, Vitamin D deficiency, tobacco dependency and the placement of a coronary arterial stent in 2017.
According to the CRF, he sustained the myocardial infarction on Oct. 27, 2020, and was diagnosed with pneumonia the following day. While both diagnoses were classified as “serious” in his CRF, they were both listed as “not related” to the vaccination, with his myocardial infection attributed to a “failed cardiac stent” and the pneumonia simply attributed to “infection.”
A female in her teens (randomization number 104650) was diagnosed with right lower extremity deep vein thrombosis on Nov. 15, 2020, which was still ongoing as of Mar. 29, 2021, the date of the CRF. She was hospitalized and her condition was classified as “serious,” but it was indicated as “not related” to the vaccine, instead attributed to a “fracture” occurring prior to her vaccination on Sept. 11, 2020.
The patient had a medical history including asthma, attention deficit hyperactivity disorder, Charcot-Marie-Tooth disease and obesity.
A male in his mid-70s (randomization number 227629) participating in the trial at Clinical Neuroscience Solutions Inc. (operating in Florida and Tennessee) sustained a series of adverse events following his vaccinations on Aug. 13 and Oct. 7, 2020.
He was diagnosed with COVID-19 on Aug. 30, 2020, which coincided with several other diagnoses classified as “serious,” including abdominal adhesions (Aug. 29, 2020), altered mental status (Aug. 29, 2020, lasting through Sept. 16, 2020), and acute hypoxic respiratory failure (Aug. 30, 2020). These diagnoses required his hospitalization.
He was also listed as having suffered from congestive heart failure on Aug. 30, 2020, but this diagnosis was listed as “not serious” and as “not related” to the vaccine, but to “prior surgery,” with no further details given. Similarly, his other serious adverse events were listed as being related to “prior” or “previous” surgery, or to “concomitant non-drug treatment.”
Other “non-serious” adverse events listed in this patient’s CRF include hypokalemia, anemia, acute renal failure, sepsis, hyponatremia, leukopenia, small bowel obstruction, aspiration pneumonia, mild concentric left ventricular hypertrophy (symptoms of which were still ongoing as of the CRF date of Mar. 29, 2021) and urinary tract infection.
The patient had a medical history encompassing ongoing hypertension, hypercholesterolemia, gastroesophageal reflux disease, constipation, hiatal hernia and previous diagnoses of small bowel resection, small bowel perforation, inguinal hernia, osteoarthritis in both knees and knee replacement (both knees).
A male in his mid-70s (randomization number 266982) participating in the trial at Boston Medical Center suffered a series of adverse events following vaccination, including pneumonia and peripheral edema. He had received two doses of the vaccine, on Oct. 2, 2020, and Oct. 27, 2020.
The patient was hospitalized for pneumonia on Jan. 20, 2021, in an event classified as “serious” but also as “not related” to the vaccine. However, the cause of his pneumonia was listed in the CRF simply as “un-related to vaccine,” while his peripheral edema diagnosis was attributed to “existing neuropathy.”
During his hospitalization with pneumonia, his blood pressure was measured as high as 179/72, with a heart rate reaching 105 beats per minute and an oxygen saturation level that fell to 92.0. In total, he had three emergency room visits during the observation period.
The patient had a medical history that included type 2 diabetes, alcoholic cirrhosis, hypothyroidism, asthma, sleep apnea, hypertension, diabetic neuropathy, congestive heart failure, generalized anxiety disorder, depression, insomnia, excessive urination, chronic obstructive pulmonary disease and HIV-positive status.
A protocol deviation also occurred involving this patient, as his diary was not activated following administration of the first dose of the vaccine.
A male in his early 40s (randomization number 68489) who participated in the trial at Cincinnati Children’s Hospital Medical Center sustained chronic myelogenous leukemia on Sept. 24, 2020, with the condition ongoing as of the date of the CRF on Mar. 29, 2021.
This was classified as a “serious” and “life-threatening” adverse event, albeit one that did not require hospitalization, but it was listed as “not related” to the vaccination but instead to a “genetic change in stem cells.”
The patient had been vaccinated on Aug. 26, 2020, and Sept. 17, 2020, and had a medical history of asthma and seasonal allergies. Other “non-serious” adverse events he sustained included leukocytosis and thrombocytosis.
A female in her mid-40s (randomization number 49018) who participated in the trial at Clinical Neuroscience Solutions Inc. was diagnosed with kidney stones on Jan. 4, 2021.
This was classified as a “serious” adverse event that required hospitalization, but was listed as “not related” to the vaccine, instead being related, again, to “kidney stone” (sic). She had received the two doses of the vaccine on Aug. 17, 2020, and Sept. 8, 2020.
The patient was diagnosed with COVID-19 on Jan. 27, 2021. Her prior medical history included migraine headaches, hypercholesterolemia and a Tarlov cyst.
A female approximately 30 years old (randomization number 53307) participating in the trial at Boston Medical Center, with nothing to report in her medical history, sustained a shoulder injury related to vaccine administration (SIRVA) on Sept. 9, 2020, with symptoms continuing until Feb. 8, 2021.
This injury was listed as being related to the second dose of the vaccine, which she received on Sept. 9, 2020 (she had previously received her first dose on Aug. 17, 2020).
A female in her late 50s (randomization number 260125) participating in the trial at Clinical Neuroscience Solutions Inc., suffered from acute exacerbation of asthma. The symptoms appeared in mid-December 2020, following her vaccination on Sept. 16, 2020, and Oct. 5, 2020.
Her symptoms were classified as serious but not life-threatening, and she was hospitalized. However, her asthma symptoms were listed as “not related” to the vaccine, instead being related to “asthma” with no further explanation provided. On Jan. 12, 2021, her blood pressure was recorded as 183/130, with a heart rate of 98 beats per minute.
Other less serious adverse events sustained by the patient included injection site pain, body pain, chills and a low-grade fever.
Her medical history included cholecystitis (and a cholecystectomy), herniated disc, total abdominal hysterectomy, bilateral oophorectomy, bilateral salpingectomy, endometriosis, hypertension, hypercholesterolemia, rheumatoid arthritis in remission, asthma, seasonal allergies, irritable bowel syndrome and obesity.
A male in his late 20s (randomization number 48413) who participated in the trial at Clinical Neuroscience Solutions Inc., sustained a bilateral pulmonary embolism on Dec. 14, 2020, with symptoms still ongoing as of the CRF date of Mar. 29, 2021.
This was listed as a “serious” adverse event that required hospitalization, but was attributed to the patient’s habit of vaping and his “sedentary lifestyle.” He had received the two doses of the vaccine on Aug. 13, 2020, and Sept. 2, 2020.
Other post-vaccination symptoms listed for the patient included fever, fatigue, headache, chills, vomiting, diarrhea, new/worsened muscle pain, new/worsened joint pain and swelling.
The patient had a medical history that included elevated triglycerides, genital herpes and seasonal allergies, in addition to a vaping habit.
The many serious adverse events – and several deaths – recorded during the Phase 3 trials are also apparent in a separate, massive document, exceeding 2,500 pages, cataloging such adverse events.
This document lists a wide range of adverse events suffered by trial participants classified as toxicity level 4 — the highest and most serious such level.
However, not one of the level 4 (most severe) adverse events listed in this particular document is classified as being related to the vaccination.
Level 4 adverse events listed in the document include but are not limited to the following, many of which occurred in multiple patients:
Acute cholecystitis
Acute respiratory failure
Adrenal carcinoma
Anaphylactic shock
Aortic valve incompetence
Appendicitis
Arrhythmia, supraventricular
Arteriosclerosis
Brain abscess
Cardiac arrest
Chronic myeloid leukemia
Complicated appendicitis/acute appendicitis with necrosis
Congenital heart disease/heart anomaly
Coronary artery occlusion
COVID-19 illness
Deep vein thrombosis
Diverticulitis
Hemiplegic migraine
Hemorrhagic stroke
Interstitial lung disease
Myocardial infarction
Orthostatic hypotension/possible postural hypotension
Osteoarthritis
Pericolic abscess
Peritoneal abscess
Renal colic
Ruptured diverticulum
Small bowel obstruction/small intestinal obstruction
Spontaneous coronary artery dissection
Subarachnoid hemorrhage
Suicidal ideation (and suicidal ideation with attempt)
Syncope
Type 2 diabetes
Worsening of abdominal pain
An “unevaluable event/“unknown of unknown origin”
Similarly, only a small number of toxicity level 3 adverse events were indicated as having been “related” to vaccination. Such adverse events included but are not limited to the following, some of which occurred in multiple trial participants:
Arthralgia
Blood glucose increase/glucose spike
Deafness/hearing loss
Dyspepsia
Hypotension
Lymph node pain
Lymphadenopathy/lymph node swelling
Musculoskeletal chest pain (non-cardiac)
Neutropenia
Pain in fingers/bilateral hands
Pruritus
Pyrexia/febrile syndrome
Severe headache
Shoulder injury related to vaccine administration
Sleep disorder/sleep disturbance
Tachycardia
Urticaria
Ventricular arrhythmia
Vertigo
Page 2,525 of the document in question also lists six trial participant deaths, with causes of death including arteriosclerosis, cardiac arrest, hemorrhagic stroke and myocardial infarction.
The small number of adverse events listed as being connected to the vaccine follows a trend noted in the previous tranche of Pfizer-BioNTech documents, released in May.
An additional document released in this month’s tranche catalogs patients who discontinued their participation in the Phase 3 trial, or whose participation was discontinued by physicians or other medical professionals.
While many patients were discontinued because they could not be located, because of a physician’s orders, because they moved to another region or for other personal reasons, numerous patients ended their participation due to adverse events, including but not limited to the following symptoms:
Acute myocardial infarction
Amnesia
Anorexia
Atrial fibrillation
Cerebral infarction
Congestive cardiac failure
Coronary artery disease
Deafness (unilateral)
Depression
Diabetic foot
Diverticular perforation
Exposure during pregnancy
Eye pain
Gait instability
Gastric adenocarcinoma
Gastrointestinal hemorrhage
Hypertension
Irregular heart rate
Loss of taste and smell
Myalgia
Paraparesis
Parkinsonism
Presyncope
Pulmonary embolism
Pyrexia
Swelling face
Tachycardia
Transient ischaemic attack
Urticaria
Vaccine allergy
Vertigo
In other instances, subjects withdrew because of fears connected to safety concerns related to the vaccine, or discomfort in receiving the second dose.
Clinical review document glosses over adverse events during trials
Also included in June’s FDA document dump was a 334-page “clinical review” document, which appears to have been approved by the FDA on Apr. 30, 2021, and which presents “pivotal data” from Phase 1/2/3 Study C4591001, conducted in the U.S., along with “supporting” Phase 1/2 data from Study BNT162-01, performed in Germany.
This document refers to both Pfizer-BioNTech vaccine, which received an EUA from the FDA, and the Pfizer Comirnaty vaccine, which received full FDA approval but is reportedly almost impossible to find at vaccination locations in the U.S.
As previously reported by The Defender, a federal judge found the Pfizer-BioNTech and Pfizer Comirnaty vaccines are legally distinct.
The clinical review document states:
“BNT162b2 has received temporary authorizations for emergency supply in 28 countries and conditional marketing authorizations in 39 countries globally.
“The name of the product supplied under emergency/temporary use authorization for all applicable regions is Pfizer-BioNTech COVID-19 Vaccine.
“The name of the product supplied under conditional marketing authorization for all applicable regions is COMIRNATY [COVID-19 mRNA Vaccine (nucleoside modified)].”
The document states that trial participants were administered one of two candidate vaccines, labeled BNT162b1 and BNT162b2 (the latter of which ultimately received an EUA from the FDA), or a placebo. A variety of dosage levels were also tested, ranging from 10 μg to 100 μg for BNT162b1, and 10 μg to 30 μg for BNT162b2.
In Phase 1 of Study BNT162-01, the clinical review reports that “40% to 45% of participants who received BNT162b1 and BNT162b2 across age groups and across dose levels reported one or more AEs [adverse events] from Dose 1 through 28 days (i.e., 1 month) after Dose 2.”
In what will turn out to be a general pattern throughout the clinical review, we are told that “most AEs were considered by the investigator as not related to study intervention and mild to moderate in severity, and all AEs were reported as resolved.”
Some specific adverse events highlighted in this part of the clinical review include:
“Among BNT162b1 recipients, 1 younger participant in the 10 μg group discontinued the study due to a moderate AE of malaise (considered as not related to study intervention) after Dose 1 and 1 younger participant in the 60 μg group discontinued due to a dose-limiting toxicity of pyrexia after Dose 1.
“One older participant in the 20 μg group had an SAE of severe syncope (considered as not related to study intervention) after Dose 1 and study treatment was withdrawn.
“Among BNT162b2 recipients, 1 younger participant in the 10 μg group discontinued the study due to a moderate AE of nasopharyngitis (considered as not related to study intervention) after Dose 1.
“One older participant in the 20 μg group had an SAE of ankle fracture (considered as not related to study intervention) after receiving both doses, was listed as recovering, and remains in follow-up.”
The clinical review also states “no deaths occurred in the Phase 1 part of Study BNT162-01.”
The review adds that “from Dose 1 of BNT162b2 30 μg to the unblinding date, 6 (50.0%) participants in the younger age group and 3 (25.0%) participants in the older age group reported at least 1 AE.”
Specifically, in this portion of the study, “two (16.7%) participants in the BNT162b2 30 μg younger age group and 1 (8.3%) participant in the BNT162b2 30 μg older age group reported at least 1 severe AE,” and “in the BNT162b2 30 μg younger age group, 3 (25.0%) participants reported at least 1 related AE and 1 (8.3%) participant reported 1 severe SAE.”
These specific adverse events, according to the review, were reported in “the system organ class (SOC) of nervous system disorders (3 [25.0%] participants in the younger age group and 1 [8.3%] participant in the older age group), followed by musculoskeletal and connective tissue disorders (1 [8.3%] participant in each age group). All AEs by preferred term (PT) were reported by no more than 1 participant.”
The review adds, “from Dose 1 to the unblinding date, 1 participant in the BNT162b2 30 μg younger age group reported a severe SAE (neuritis) that was assessed by the investigator as not related to study intervention,” and “there were no Phase 1 participants randomized to BNT162b2 30 μg or corresponding placebo who died through the data cutoff date of 13 March 2021.”
Review of results from Study C4591001
While “incidences in the BNT162b2 and placebo were similar within the age groups for younger (9.1% vs 11.1%) and older (4.3% vs 8.9%) participants, among those who received BNT162b2 instead of the placebo, “two severe events of myalgia and gastric adenocarcinoma (which was also an SAE) were reported for 2 participants in the … younger age group, both assessed by the investigator as not related to study intervention.”
It is further mentioned that “the only discontinuation due to an AE during this time was the participant in the BNT162b2 younger age group who reported an SAE of gastric adenocarcinoma (discontinued from the study on Day 23 after Dose 1 of BNT162b2).”
Ultimately, from dose 1 to 1 month after dose 2 for participants during the blinded safety follow-up of study C4591001, “the numbers of overall participants who reported at least 1 AE and at least 1 related AE were higher in the BNT162b2 group (30.2% and 23.9%, respectively) as compared with the placebo group (13.9% and 6.0%, respectively).”
Specifically, “severe AEs were reported by 1.2% and 0.7% in in the BNT162b2 and placebo groups respectively, and life-threatening AEs were similar (0.1% in both groups),” and “SAEs and “AEs leading to withdrawal were reported by ≤0.6% and ≤0.2%, respectively, in
both groups,” while “discontinuations due to related AEs were reported in 13 participants in the BNT162b2 group and 11 participants in the placebo group (0.1% in both groups).”
Overall, as reported for this part of the study, “in the younger age group, the number of participants who reported at least 1 AE from Dose 1 to 1 month after Dose 2 was 4233 (32.6%) and 1871 (14.4%) in the BNT162b2 and placebo groups, respectively. In the older age group, the number of participants who reported at least 1 AE from Dose 1 to 1 month after Dose 2 was 2384 (26.7%) and 1177 (13.2%) in the BNT162b2 and placebo groups, respectively.”
The review specifies that “the most frequently reported AEs in the BNT162b2 group … were injection site pain (2915 [13.3%]), pyrexia (1517 [6.9%]), fatigue (1463 [6.7%]), chills (1365 [6.2%]), headache (1339 [6.1%]), and myalgia (1239 [5.7%]),” however, some more serious adverse events that were reported during this stage of the trial included facial paralysis, cardiac disorders, hepatic cirrhosis, cholecystitis/cholecystitis acute, biliary colic, bile duct stone, biliary dyskinesia, lymphadenopathy, appendicitis, optic neuritis and hypersensitivity/anaphylaxis.
Overall, according to the review, “from Dose 1 to 1 month after Dose 2, severe AEs reported during the blinded follow-up period were low in frequency, reported in 1.2% of BNT162b2 recipients and 0.7% of placebo recipients.”
During the “open-label follow-up period,” referring to the period when the initial trial has been completed but participants are invited to continue taking the study drug for an additional period, the review states “three participants originally randomized to BNT162b2 died during open-label follow-up.”
While one of these deaths was reportedly due to a road accident, the other two were attributed to lung metastases and myocardial infarction. However, none of these deaths “were assessed by the investigator as related to study intervention.”
Furthermore, according to the report, during this period “there were 12,006 participants who had at least 6 months of follow-up. Among these, 3,454 participants (28.8%) reported at least 1 AE and 2245 participants (18.7%) reported at least 1 related AE. Severe AEs and SAEs were reported by 2.1% and 1.6%, respectively.”
The review provides data for participants from dose 3 (first dose of BNT162b2) to the data cutoff date. The severe adverse event incidence rate (IR) was 6.0 per 100 PY (patient-years), with specific conditions reported including pulmonary embolisms, thrombosis, urticaria, a cerebrovascular accident and COVID-19 pneumonia.
Here, the review adds that the IR for original placebo participants who had at least 1 life-threatening AE from Dose 3 to the data cutoff date was 0.5 per 100 PY. Only one such life-threatening event, an instance of anaphylactoid reaction, was considered to be related to the vaccination. Other life-threatening, serious adverse events included cardio-respiratory arrest, gastrointestinal necrosis, deep vein thrombosis and pulmonary embolism.
The report also notes, “There were 15 deaths in the BNT162b2 group and 14 deaths in the placebo group from Dose 1 to the unblinding date during the blinded placebo-controlled follow-up period.”
However, the report does not appear to go into detail about the causes of death for either group, other than to state, “None of these deaths were assessed by the investigator as related to study intervention.”
In the “Blinded Follow-Up Period from Dose 1 Through 1 Month After Dose 2,” in the BNT162b2 group, “SAE [serious adverse events] was similar in the BNT162b2 group (0.6%) and in the placebo group (0.5%),” with three SAEs in the non-placebo group deemed to be related to the vaccine. These included ventricular arrhythmia, lymphadenopathy and SIRVA.
During the “open-label follow-up period” for “original BNT162b2 participants,” the report states “one younger participant with no past medical history had a life-threatening SAE of myocardial infarction 71 days after Dose 2 that was assessed by the investigator as related to study intervention.”
However, despite its life-threatening nature, this condition “lasted 1 day and resolved the same day.”
Overall, “from Dose 1 to 6 months after Dose 2, during the blinded and open-label follow-up periods, 190 (1.6%) participants in the BNT162b2 group reported at least 1 SAE,” and “the number of participants who reported at least 1 SAE was 73 (1.1%) and 117 (2.2%) in the younger and older age groups, respectively.”
These SAEs were categorized as neoplasms, infections and infestations, gastrointestinal disorders, hepatobiliary disorders, respiratory/thoracic/mediastinal disorders and injury/poisoning/procedural complications.
An original placebo participant who received BNT162b2 for Dose 3 experienced a severe adverse event that “was assessed by the investigator as related to study intervention; specifically, “an anaphylactoid reaction 2 days post Dose 3” leading to the participant’s withdrawal from the study, despite a reported resolution.
A separate subsection in the report specifically addressed cases of Bell’s palsy and facial paralysis among trial participants. Specifically, “during the blinded placebo-controlled follow-up period, 6 participants developed one-sided facial paralysis (Bell’s palsy): 4 were randomized to BNT162b2 (all male) and 2 were randomized to placebo (1 male; 1 female),” according to the review.
Regarding the four vaccinated trial participants, their ages ranged from 40 to 70, with symptoms appearing three to 48 days after their last dose. Their symptoms were recorded as “mild to moderate in severity,” with duration ranging “from 3 to 68 days,” and with two of these cases “considered by the investigator to be related to study intervention.”
SNIP article a bit too long to post in one posting. Important to read all at original source
-
-
26th June 2022, 09:43 AM
#3326
Iridium

Re: Coronavirus
Dachsie comment:
In response to posting #3325 earlier today, Dachsie deems the following deserving of the MOC award (Most Outrageous Case).
"• A male in his mid-70s (randomization number 227629) participating in the trial at Clinical Neuroscience Solutions Inc. (operating in Florida and Tennessee) sustained a series of adverse events following his vaccinations on Aug. 13 and Oct. 7, 2020.
He was diagnosed with COVID-19 on Aug. 30, 2020, which coincided with several other diagnoses classified as “serious,” including abdominal adhesions (Aug. 29, 2020), altered mental status (Aug. 29, 2020, lasting through Sept. 16, 2020), and acute hypoxic respiratory failure (Aug. 30, 2020). These diagnoses required his hospitalization.
[/COLOR]
He was also listed as having suffered from congestive heart failure on Aug. 30, 2020, but this diagnosis was listed as “not serious” and as “not related” to the vaccine, but to “prior surgery,” with no further details given. Similarly, his other serious adverse events were listed as being related to “prior” or “previous” surgery, or to “concomitant non-drug treatment.”
Other “non-serious” adverse events listed in this patient’s CRF include hypokalemia, anemia, acute renal failure, sepsis, hyponatremia, leukopenia, small bowel obstruction, aspiration pneumonia, mild concentric left ventricular hypertrophy (symptoms of which were still ongoing as of the CRF date of Mar. 29, 2021) and urinary tract infection."
-
-
26th June 2022, 01:11 PM
#3327
Iridium

Re: Coronavirus

https://www.bitchute.com/video/6QC3KGmvTyiF/
Miss BRAZIL (27yo Gleycy Correria) DEAD After Suffering Brain Hemorrhage & HEART ATTACK
Miss Brazil, Age 27, suffers brain hemorrhage and heart attack after tonsillectomy.
Died after a tonsillitis operation? Hmmm....OK. 27yo Miss Brazil: Gleycy Correria - Dead After Suffering A Brain Hemorrhage & Heart Attack ....and YES she was quaxxinated.
-
-
26th June 2022, 03:49 PM
#3328
Iridium

Re: Coronavirus
https://www.americaoutloud.com/the-w...ew-sars-cov-2/
The Warning Signs Make Monkeypox the New SARS-CoV-2
by Peter Breggin MD and Ginger Ross Breggin | Jun 26, 2022 | Health, Politics, World

My mom used to always say, “practice makes perfect.” Despite complaining, my siblings and I learned that doing something repeatedly develops judgment, skills, knowledge, good instincts, and a much better final product. Our products improved when we learned to evaluate our mistakes and not repeat them. So, let’s consider how we are doing two and a half years after the advent of COVID.
A New Disease Outbreak
Pride month has been declared for June in the United States, and Pride is celebrated in many parts of the world, in parades and great gatherings, and parties in the US, Europe, and other locations. Hundreds of thousands are celebrating.1
Unfortunately, there is an outbreak of monkeypox that has specifically been infecting Pride participants–“men who have sex with men,” –as the legacy media put it. The outbreak was first reported in May when a single case was detected in the UK.2
Scientists are now reporting that this outbreak of monkeypox is mutating at an unprecedented, accelerated rate compared to other outbreaks and is the “largest monkeypox virus (MPXV) outbreak described so far in non-endemic countries.3 Ever. As of June 23rd, more than 3,417 identified cases were reported across fifty-eight countries on multiple continents.4
Is Monkeypox a PHEIC (pronounced FAKE) Yet?
The World Health Organization (WHO) just called a meeting to determine if the monkeypox outbreak should be classified as a Public Health Emergency of International Concern.5 The emergency status “known as PHEIC — pronounced ‘fake’ — applies to an extraordinary event that carries a public health risk via the international spread of a disease, and one that potentially requires a coordinated response.”6 The last time WHO called a meeting to consider classifying a disease as a PHEIC was in January of 2020, when they evaluated the SARS-CoV-2 outbreak. WHO declined to label the COVID-19 disease a PHEIC, changing their mind just a week later.
And I am sorry, but seriously…. the pronunciation of the emergency status label that was applied to SARS-CoV-2 and now considered for monkeypox is pronounced FAKE You can’t make this stuff up…and I didn’t.
You can’t make this stuff up…and I didn’t.
The timing of this monkeypox outbreak is especially unfortunate because the enormous numbers of group gatherings to celebrate Pride month bring together “men who have sex with men” in exuberant celebrations, and human nature often takes its course.
Monkeypox is spread by skin-to-skin contact, especially of an intimate nature. WHO reports that the “monkeypox virus is transmitted from one person to another by close contact with lesions, body fluids, respiratory droplets, and contaminated materials such as bedding. The incubation period of monkeypox is usually from 6 to 13 days but can range from 5 to 21 days.”7
Despite the mass gatherings planned around the world to celebrate Pride month, the CDC, NIH, and other public health officials refuse to clearly and broadly state that there should be no intimate contact of any kind between those at risk for this disease or infected during this outbreak.8 Gay and bisexual men need to protect themselves and each other for a few weeks, so they do not get sick. Our friend and colleague, Paul Alexander, Ph.D., has very plainly called out these public officials for their dereliction of duty in failing to warn affected communities clearly about how they can protect themselves and each other.9
In November 2021, a report was issued by the Nuclear Threat Initiative, which, along with the Munich Security Conference, held a tabletop exercise to consider responses to a fictional monkeypox outbreak that was posited to occur in May 2022.10
Stretching credulity, fiction has now been replaced by fact as the world experiences a genuine explosion of the monkeypox disease starting on the exact assigned date. Could this exact prediction be one more big coincidence?
Earlier Prescient Pandemic Predictions
All the way back to 2010 and earlier, there have been tabletop exercises making predictions and running drills on how to handle an emerging pandemic. The pandemic simulation exercise at Johns Hopkins Center for Health Security in November of 2019 predicted an outbreak of a coronavirus.11 Two months later, in January 2020, the first reports of a coronavirus outbreak—Sars-CoV-2—circulated. Now we have had a 2021 tabletop exercise positing an outbreak of monkeypox, predicting an outbreak would occur in May of this year, and here it is, right on schedule.
Lessons from COVID-19
During the early days of COVID, delays in implementing containment measures stretched on for weeks. Hundreds of flights left China daily for the four corners of the earth, carrying infected individuals to many countries, including thousands of returning foreign students to the US. When President Trump suggested that flights from China should be shut down, he was charged with xenophobia.12
Sadly, we failed to learn the lesson of containment and are seeing a repeat with the appearance of monkeypox. Abstinence, the most logical step for containing monkeypox, has not been recommended by the authorities.
CDC, NIH, and other public health authorities fail to take the scientific and medically sound action of advising abstinence and stand by as another plague descends upon the gay community. Instead, there have been multiple official reassurances that monkeypox is not usually contagious and that a vaccine already exists.13
Are we Really this Naïve?
Can we be so naïve as to believe it is chance when two times out of two, there have been practice runs of a mythical epidemic, and then that specific epidemic appears within months? And both times, there are botched efforts to contain the disease when the outbreak is identified, resulting in a worldwide spread of the pathogen. And each time, the pathogen, first Sars-CoV-2 in 2020, and now this strain of monkeypox is unique in its behavior.
Like coronaviruses, viruses related to smallpox, mousepox, monkeypox, cowpox, and others have been the focus of laboratory manipulation and gain of function (GOF) research since at least 2003. Again like coronaviruses, these pathogens are common in the biocontainment labs of the world.14
But…There are Vaccines!
Conveniently, COVID mRNA and DNA vaccines were already in development for some time prior to the SARS-CoV-2 outbreak. And now, as it turns out, we have monkeypox vaccines handy. These vaccines are also used to inoculate against smallpox, which has been eradicated since 1980.15
New York City opened the first clinic for monkeypox vaccines just this week to administer doses. The clinic has already had to cancel further walk-in appointments because, within 24 hours of opening the clinic, they were booked out into the following week.16 The New York City Health Commissioner reports anxiety to be high, especially among sexually active gay and bisexual men, and so the city decided to make the vaccines available.
Cardiac Adverse Events of Special Interest
The two-dose JYNNEOS vaccine administered 28 days apart is a smallpox and monkeypox vaccine.17 The package insert for the JYNNEOS vaccine contains a section on “Cardiac Adverse Events of Special Interest.”18 This raises concern among doctors tracking the alarming number of COVID vaccine cardiac side effects.19 If individuals already have developed cardiac adverse events from COVID vaccines, they need to avoid another vaccine with the same possible adverse effect.
Individuals who have already had a COVID vaccine, and especially anyone who has experienced cardiac complications following a COVID vaccine, need to consider the greater cardiac event risk of a second vaccine exposure when deciding whether to be vaccinated for monkeypox.20 Few hospitalizations and no deaths have been reported for the current outbreak “outside of an endemic country.”21 Business is picking up for the vaccine manufacturer, however.
Mistakes were Made, But No One is Learning
Despite tabletop exercises for COVID and now monkeypox, mistakes are being repeated. Tabletop exercises without emphasis on correcting mistakes make perfect sense only if one is preparing to spread a little plague chaos. The predators have got to have some “practice runs” to ensure everyone is genned in on the complexities of the execution. The propagandists, the social psychologists, the legacy media, the various agencies, the chosen, anointed corporations (Pfizer and Moderna in the case of COVID in the US) …. well, it takes tremendous coordination.
If preventing the spread of disease and helping the sick is the goal, these exercises continue to fail.
What if the goal is vaccines for money and chaos?
Look at what had to happen with COVID in order to achieve the release of the vaccines. Jack up the numbers of COVID deaths by sending infected elders back to nursing homes (NY and NJ, among other states). Suppress successful FDA-approved medications as early treatments like hydroxychloroquine and ivermectin so that the Emergency Use Authorizations could be approved for the vaccines. Manipulate the research to show positive outcomes for vaccines and suppress negative outcomes of the COVID vaccines in trials.22,23 Details of much of the manipulation and the complexities are addressed in our book COVID-19 and the Global Predators: We are the Prey.
I find that using the data we unearthed for our book and continuing to look for the predator connections in chaos further illuminates our current circumstances and removes much of the mystery. The food shortages, energy “shortages,” disruptions in schools, and so many seemingly random actions are explained. Chief among the alarming circumstances are rumors that cash–folding money and coin around the world–will be canceled, and we will all be forced to convert to digital currency and digital wallets. Vaccine passports—in high demand by controlling governments since the COVID pandemic– are one vector being employed to introduce more widely applicable digital wallets. See our column at America Out Loud on why each of us who values freedom must use physical cash instead of digital money, every chance we get.24
There are no Coincidences
This much chaos, this much random disaster in so many sectors, does not just happen. Repeating the same mistakes made in the pandemic that occurred less than three years ago is not just ignorance. We are not just experiencing a streak of bad pandemic luck and massive chaos in other sectors.
There is a huge amount of planning and outright manipulation for so many systems to go so wrong so fast at the same time, both medically and in public health and in other sectors that are being severely disrupted.
What if the deeper purpose of the pandemics is to weaken the nation-states of the world and enable a global-level cabal of private predator corporations, narcissistic billionaires, and the Chinese Communist government to seize world governance.25 A recent and ongoing example of this is the World Health Organization’s attempts to seize enforceable global control of all nations’ health systems and emergency operations when WHO arbitrarily decides it is necessary.26
We cannot afford to be complacent or naïve anymore. Predators exist, and they are stalking us and our democratic republics. Are we going to continue to be their prey until we are too weakened to resist?
Author’s Note: The author wants to acknowledge Gatito Bueno, of the substack “Bad Cattitude” for his article “kitten corner: hey, hey it’s the monkeypox.”27 Gatito Bueno’s substack inspired my initial thoughts and comments on that column and thus this column.
Primary author Ginger R. Breggin. Peter R. Breggin MD and Ginger Breggin are the authors of the bestselling new book, COVID-19 and the Global Predators: We are the Prey, with introductions by top COVID-19 scientists and physicians, Peter A. McCullough MD, MPH; Elizabeth Lee Vliet MD; and Vladimir “Zev” Zelenko MD. Over 100,000 sold.
References:
1 European festivals should not be scrapped due to monkeypox: WHO (yahoo.com)
2 Monkeypox mutates at unprecedented rate, study finds (nypost.com)
3 Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus | Nature Medicine
4 Pandemic or Epidemic: WHO Monkeypox Announcement Set for Today (newsweek.com)
5 WHO Monkeypox Meeting: What Is an Emergency of International Concern? (newsweek.com)
6 How Serious is Monkeypox? WHO Says Not Global Emergency – Bloomberg
7 Multi-country monkeypox outbreak in non-endemic countries (who.int)
8 Monkeypox | Poxvirus | CDC
9 WARNING: CDC & public health officials & WHO must stop the pandering to the gay community & state just facts, where the risk is, how the risk happens & how to stop it; NOT side step & ENCOURAGE it (substack.com)
10 Strengthening Global Systems to Prevent and Respond to High-Consequence Biological Threats (nti.org)
11 Pandemic simulation exercise spotlights massive preparedness gap | Hub (jhu.edu)
12 After attacking Trump’s coronavirus-related China travel ban as xenophobic, Dems and media have changed tune | Fox News
13 You can be worried about monkeypox if you want to – Vox
14 US develops lethal new viruses | New Scientist
15 Smallpox Questions and Answers: The Disease and the Vaccine (ny.gov)
16 NYC Monkeypox Cases Jump 30% Amid Rush on Limited Vaccine Supply (msn.com)
17 Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for Preexposure Vaccination of Persons at Risk for Occupational Exposure to Orthopoxviruses: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022 | MMWR (cdc.gov)
18 Package Insert – JYNNEOS (fda.gov)
19 In memory of those who “died suddenly” in the United States and worldwide, June 14-June 20 (substack.com)
20 Cardiac complications following mRNA COVID-19 vaccines: A systematic review of case reports and case series – PubMed (nih.gov)
21 Global monkeypox outbreak isn’t an international public health emergency ‘at present,’ WHO rules | Fortune
22 WARNING! Pfizer Lied About Results in COVID-19 Vaccine Trials for Babies and Toddlers – Vaccine Impact
23 Serious Adverse Events of Special Interest Following mRNA Vaccination in Randomized Trials by Joseph Fraiman, Juan Erviti, Mark Jones, Sander Greenland, Patrick Whelan, Robert M. Kaplan, Peter Doshi :: SSRN
24 https://www.americaoutloud.com/rescu...gital-slavery/
25 The Inhumanity and Treachery of Governments – Lessons of COVID – America Out Loud
26 Freedom Fighters Stop Dictatorship of World Health Organization – America Out Loud
27 kitten corner: hey, hey it’s the monkeypox (substack.com)
Peter Breggin MD and Ginger Ross Breggin
Peter Breggin MD and Ginger Ross Breggin
Peter Breggin MD and Ginger Ross Breggin have been married and working together for almost 40 years. Peter is known as "The Conscience of Psychiatry" for his many decades of successful reform work in mental health. He has published more than 20 medical and popular books, several coedited or coauthored by Ginger, including the huge bestseller Talking Back to Prozac. He has written more than 70 peer-reviewed publications and testified in court more than 100 times with many cases related to drug company and medical malfeasance. The couple has now turned their attention to the misuse of science and the suppression of freedoms surrounding COVID-19 and its origins by those they identify as "global predators."
Peter and Ginger have written the bestselling new book, COVID-19 and the Global Predators: We are the Prey with introductions by top COVID-19 scientists and physicians, Peter A. McCullough MD, MPH; Elizabeth Lee Vliet MD; and Vladimir "Zev" Zelenko. The book is available everywhere.
-
-
26th June 2022, 05:57 PM
#3329
Iridium

Re: Coronavirus
CDC Investigates Rare Disease in Florida Dubbed as ‘One of the Worst Outbreaks Among Gay and Bisexual Men in U.S. History’
The U.S. Centers for Disease Control and Prevention (CDC) announced on Wednesday that the agency continued collaborating with the Florida Department of Health to investigate a rare disease outbreak among gay and bisexual men.
In a press release, the CDC said they are assisting with the meningococcal disease outbreak investigation in Florida that spread among homosexual males, including those with HIV.
According to the agency, this is one of the worst outbreaks of meningococcal disease among gay and bisexual men in U.S. history.
CDC is now urging homosexuals to get a MenACWY vaccine if they reside in Florida or by contacting their doctor’s office, pharmacy, community health center, or local health department or their healthcare provider about the vaccination if traveling to Florida.
-
-
26th June 2022, 06:08 PM
#3330
Iridium

-
 Posting Permissions
Posting Permissions
- You may not post new threads
- You may not post replies
- You may not post attachments
- You may not edit your posts
-
Forum Rules

![[Most Recent Quotes from www.kitco.com]](http://www.kitconet.com/images/sp_en_6.gif)



 Reply With Quote
Reply With Quote



 You can’t make this stuff up…and I didn’t.
You can’t make this stuff up…and I didn’t.